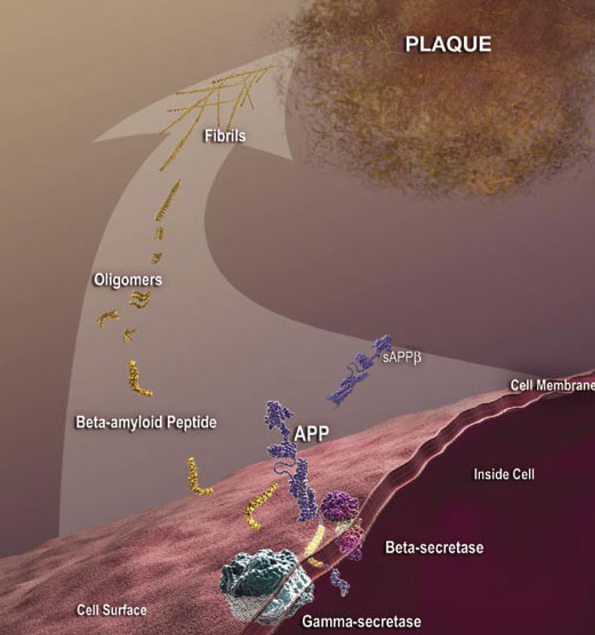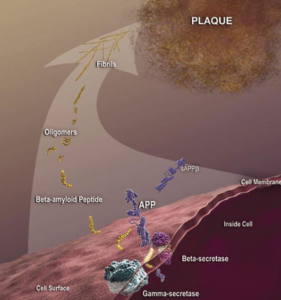
Although some antibodies for passive immunization are in clinical trials, passive immunization is not the only potential intervention strategy based on toxic Aβ and other antibodies may be more effective as passive immunizing agents. A modified form of Aβ that has a cyclized form of glutamate at the end (pyroglutamate) is particularly toxic to neurons (3) and is present in deposits of Aβ in the cerebral blood vessels and in the brain. This form of Aβ with pyroglutamate (AβpE3) is antigenic; antibodies can be generated that recognize this form specifically. Tests of these AβpE3 -targeted antibodies in animal models of Alzheimer’s disease through a passive immunization approach reduce neuron loss and improve the performance of the mice in memory tests (4, 5).
Not only could AβpE3 be a good target for antibodies used for passive immunization, but researchers (6) are investigating the potential of using this modified protein as a self-antigen in an active immunotherapy approach (similar to an antigen in a vaccine). Injecting mice that are a model of Alzheimer’s disease with the antigen preparation containing AβpE3-15 (a 12-amino-acid peptide from Aβ with pyroglutamate as the first residue) reduced Aβ accumulation and prevented memory impairment in the mice. Another group (7) is testing inhibitors of glutaminyl cyclase, the enzyme that mediates the formation of the cyclized glutamate at the end of proteins and peptides, as a potential strategy to treat Alzheimer’s disease. Although still in the “chemistry” phase of rational design and not tested in animals, these may have clinical application especially if the inhibitors show selectivity for the ability of this enzyme to modify Aβ and do not interfere with the cyclization of other substrates.
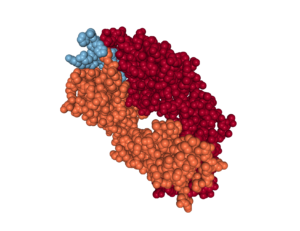
One of the groups (5) investigating alternative antibodies for passive immunization therapy performed structural analysis of the antibodies bound to AβpE3-42. This analysis revealed that some of the antibodies had the peptide buried in a deep pocket (Figure 2), which could be important for developing additional antibodies for passive immunization. Additionally, the structural analysis revealed a potential molecular mechanism for the enhanced aggregating properties and degradation resistance of AβpE3-42 compared to Aβ fragments of the same composition lacking only the pyroglutamate modification. Understanding these properties could lead researchers into new directions for intervention.
Treating patients after symptoms have appeared has not been very successful; whereas studies with mice engineered to develop Alzheimer’s disease show that early intervention can be effective in reducing memory impairment and onset of symptoms. However, initial screening of potential therapeutics in mice is not cost effective or humane. Some groups are developing new assays for rapidly screening potential therapeutics. For example, Lin and colleagues (8) used cultured cortical neurons and a voltage-sensitive fluorescent dye (DiBAC4) to screen for natural products in plants or herbal medicines that inhibited Aβ-stimulated depolarization (activation) of the neurons.
Baicalein was one of the molecules present in the herbal medicine (Figure 3). This molecule has been previously identified as improving memory in a mouse model of Alzheimer’s disease. Applying pure baicalein to the neurons inhibited Aβ-stimulated depolarization, reduced Aβ-mediated activation of a stress-activated kinase JNK, and reduced the appearance of markers of cell death by apoptosis. Baicalein also interfered with pharmacological activation of glutamate receptors of the AMPA and NMDA classes, which may be important for the mechanism of action as an inhibitor of Aβ-stimulated neuronal depolarization. Although baicalein had already been known as a molecule that could be useful in treating Alzheimer’s disease in mouse models, identification of this molecule validates the usefulness of this screen to identify candidates for further analysis.
Even considering only Americans, the current number of people with Alzheimer’s disease exceeds 5 million and this is predicted increase in the next decades to more than 13 million in the United States alone (9). Thus, this disease is and will continue to be a major health care cost and burden. Methods to detect susceptible people well in advance of symptoms, effective and rapid screening approaches for developing new therapies, and ongoing research into the basic mechanisms underlying the disease, especially at the early stages, are all needed.
Highlighted Research
- Lazarevic, V., Fieńko, S., Andres-Alonso, M., Anni, D., Ivanova, D., Montenegro-Venegas, C., Gundelfinger, E.D., Cousin, M.A., Fejtova, A., Physiological concentrations of amyloid beta regulate recycling of synaptic vesicles via alpha7 acetylcholine receptor and CDK5/calcineurin signaling. Front. Mol. Neurosci. 10, 221 (2017). PubMed
- Sevigny, J., Chiao, P., Bussière, T., Weinreb, P.H., Williams, L., Maier, M., Dunstan, R., Salloway, S., Chen, T., Ling, Y., O’Gorman, J., Qian, F., Arastu, M., Li, M., Chollate, S., Brennan, M.S., Quintero-Monzon, O., Scannevin, R.H., Arnold, H.M., Engber, T., Rhodes, K., Ferrero, J., Hang, Y., Mikulskis, A., Grimm, J., Hock, C., Nitsch, R.M., Sandrock, A., The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56 (2016). PubMed
- Gunn, A.P., Wong, B.X., Johanssen, T., Griffith, J.C., Masters, C.L., Bush, A.I., Barnham, K.J., Duce, J.A., Cherny, R.A., 2016. Amyloid-β peptide Aβ3pE-42 induces lipid peroxidation, membrane permeabilization, and calcium influx in neurons. J. Biol. Chem. 291, 6134–6145 (2016). PubMed
- Antonios, G., Borgers, H., Richard, B. C., Brauß, A., Meißner, J., Weggen, S., Pena, V., Pillot, T., Davies, S.L., Bakrania, P., Matthews, D., Brownlees, J., Bouter, Y., Bayer, T.A., Alzheimer therapy with an antibody against N-terminal Abeta 4-X and pyroglutamate Abeta 3-X. Sci. Rep. 5, srep17338 (2015). PubMed
- Piechotta, A., Parthier, C., Kleinschmidt, M., Gnoth, K., Pillot, T., Lues, I., Demuth, H.-U., Schilling, S., Rahfeld, J.-U., Stubbs, M.T., Structural and functional analyses of pyroglutamate-amyloid-β-specific antibodies as a basis for Alzheimer immunotherapy. J. Biol. Chem. 292, 12713–12724 (2017). PubMed
- Li, G., Hu, Z.-W., Chen, P.-G., Sun, Z.-Y., Chen, Y.-X., Zhao, Y.-F., Li, Y.-M., Prophylactic vaccine based on pyroglutamate-3 amyloid β generates strong antibody response and rescues cognitive decline in Alzheimer’s disease model mice. ACS Chem. Neurosci. 8, 454–459 (2017). PubMed
- Hoang, V.-H., Tran, P.-T., Cui, M., Ngo, V.T.H., Ann, J., Park, J., Lee, Jiyoun, Choi, K., Cho, H., Kim, H., Ha, H.-J., Hong, H.-S., Choi, S., Kim, Y.-H., Lee, J., 2017. Discovery of potent human glutaminyl cyclase inhibitors as anti-Alzheimer’s agents based on rational design. J. Med. Chem. 60, 2573–2590 (2017). PubMed
- Lin, T.-S., Tsai, H.-J., Lee, C.-H., Song, Y.-Q., Huang, R.-S., Hsieh-Li, H.-M., Liang, M.-C., Lin, Y., An improved drugs screening system reveals that baicalein ameliorates the Aβ/AMPA/NMDA-induced depolarization of neurons. J. Alzheimers Dis. 56, 959–976 (2017). PubMed
- Alzheimer’s Association, 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 12, 459–509 (2016). PubMed
Highlighted Therapies
Crenezumab, Alzforum http://www.alzforum.org/therapeutics/crenezumab (accessed on 6 September 2017).
Gantenerumab, Alzforum http://www.alzforum.org/therapeutics/gantenerumab (accessed on 6 September 2017).
Aducanumab, Alzhforum http://www.alzforum.org/therapeutics/aducanumab (accessed on 6 September 2017).
Cite as: N. R. Gough, Targeting Pyroglutamate Aβ in Alzheimer’s Disease. BioSerendipity (6 September 2017) https://www.bioserendipity.com/2017/09/06/targeting-pyroglutamate-a%ce%b2-in-alzheimers-disease/.