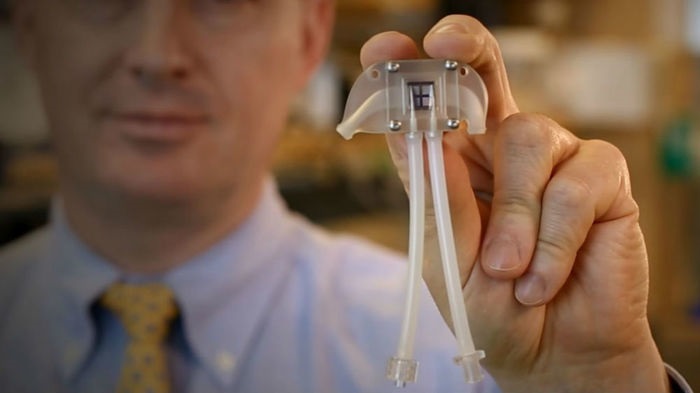
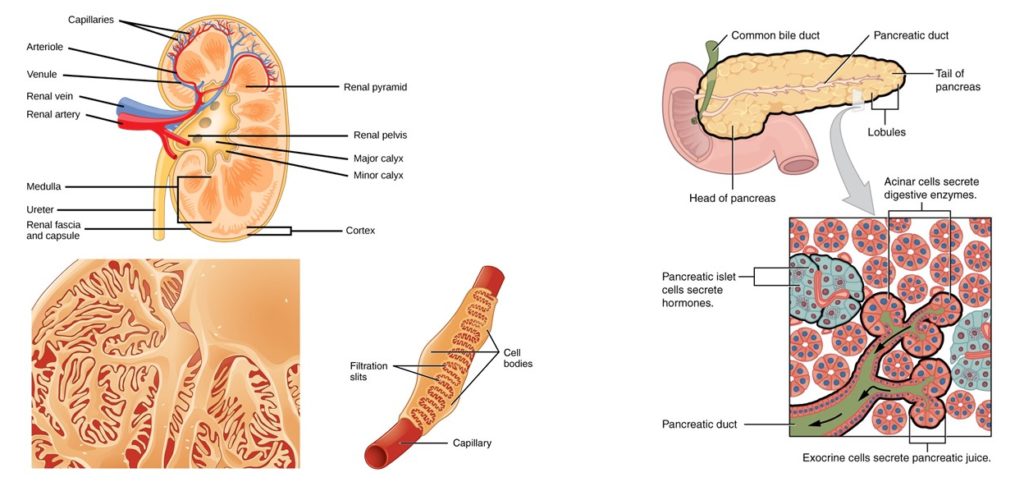
One of the challenges to building artificial organs is finding or creating suitable materials for use in such devices. If the device contains living cells, then those cells need to receive nutrients from, exchange gases with, and send cellular waste products into blood. In addition, the cells should also respond appropriately to circulating regulatory signals, such as hormones, and release regulatory molecules into the circulation. Efforts are underway to develop implantable artificial kidney devices and pancreas devices to replace the function of these organs (Figure 1). To replace dialysis for people with end-stage renal disease (ESRD), the cells need to filter the blood for molecules that need to be eliminated from the body. To replace lost pancreas function, the cells need to detect glucose and release insulin into blood.
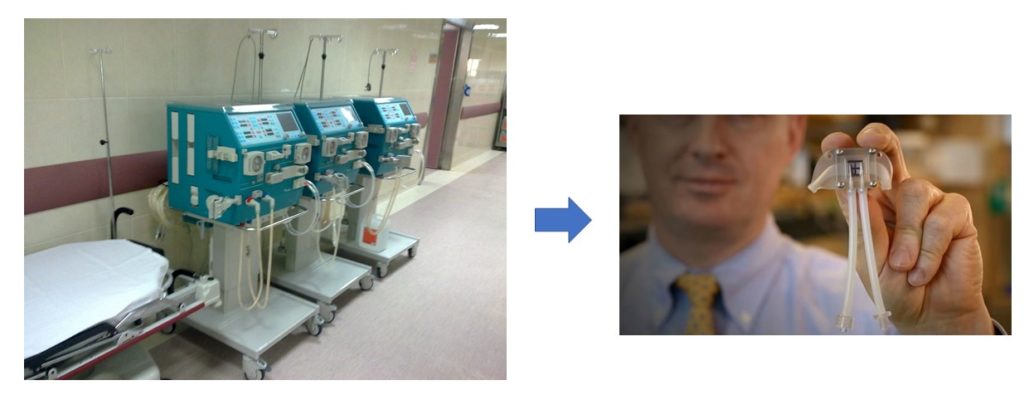
For artificial kidneys, the ideal arrangement utilizes the pressure of the heart beat to push blood through the membrane and into contact with the kidney cells in the artificial kidney device. This means that the artificial kidney device needs to be placed within the circulatory system, where it must withstand the hemodynamic pressures associated with normal variations in blood pressure. Additionally, the device cannot trigger blood clots. Shuvo Roy, a researcher in the Department of Bioengineering and Therapeutic Sciences, Schools of Pharmacy and Medicine, University of California, San Francisco (UCSF), and William Fissell, a medical doctor at Vanderbilt University Medical Center, Tennessee, are leading The Kidney Project (1) to develop such a device (Figure 2)
The second study with the pigs was performed to test a different manufacturing process for the silicon nanopore membranes with the goal of increasing the movement of molecules (diffusion) across the membrane. This process introduced a pattern to the backside, which contained the supporting material, to reduce the thickness of this support structure and enhance diffusion across the membrane. After testing diffusion using mechanical pumps, the authors attached pigs to the device as an external blood filter and collected the fluid filtered from the blood over 6 hours. The results showed an improvement over previous designs in diffusion rates and clearance of molecules that are filtered by the kidneys from the blood.
Future work will have to test alternative coatings to PEG and resolve the problem of detachment of the membrane from the support. However, these studies show the potential for advances in materials science to lead to “a surgically implantable, self-monitoring, and self-regulating bioartificial kidney” (1).
The second application of silicon nanopore membranes is quite different. In this application, the silicon nanopore membrane prevents the immune system from destroying transplanted cells. Type 1, or insulin-dependent diabetes, is caused by autoimmune attack on the β cells of the pancreas. These are the cells that produce insulin. Thus, patients with type 1 diabetes lack the cells that produce insulin/ Because the immune system targets these cells for destruction, transplantation is not a viable solution. Roy and Fissell (4, 5) are exploring the use of silicon nanopore membranes to surround the pancreas cells isolated from islets (the places in the pancreas where β cells can be found) and protect them from the toxic effects of immune cells and molecules produced by immune cells, such as pro-inflammatory cytokines. In the first study, the researchers (4) showed that the membranes had a high rate of diffusion, allowing the passage of glucose and insulin while limiting the passage of pro-inflammatory cytokines. Cytokines are much smaller than immune cells, so these membranes will also prevent the immune cells from destroying cells surrounded by these membranes. When mouse islet cells were placed between two silicon nanopore membranes, the cells were protected from the toxic effects of the cytokines added to the medium and the cells released insulin in response to the addition of glucose to the medium.
In the second study, the researchers (5) placed a bioartificial pancreas (BAP) device into pigs. Similar to the strategy for the artificial kidney, the device was connected to the circulation. Different from the artificial kidney studies, which only tested a device containing the silicon nanopore membrane without living cells, the BAP devices contained islet cells. The cells in the BAP were exposed to the molecules in the blood that could pass through the membrane in response to the pressure associated with the heartbeat. Over the 3 days of the experiment, device maintained flow and most of the islet cells survived. This short-term study provides proof-of-principle for protecting cells from immune attack using silicon nanopore membranes.
Along with progress in growing functional tissues and organoids in culture, the ability to manufacture selectively permeable membranes that are compatible for implantation (biocompatible) will lead to exciting new treatments options for patients suffering from various types of organ failure or autoimmune tissue destruction. The concept of a immunoprotective membrane for transplanted tissue may also reduce or eliminate the need for immunosuppressive therapy and expand the options for some transplant recipients.
Highlighted Articles and Resources
- The Kidney Project. USCF, Schools of Pharmacy and Medicine, Department of Bioengineering and Therapeutic Sciences. https://pharm.ucsf.edu/kidney (accessed on 11 September 2017)
- Kensinger, C., Karp, S., Kant, R., Chui, B.W., Goldman, K., Yeager, T., Gould, E.R., Buck, A., Laneve, D.C., Groszek, J.J., Roy, S., Fissell, W.H., First implantation of silicon nanopore membrane hemofilters. ASAIO J. 62, 491–495 (2016). doi:10.1097/MAT.0000000000000367 PubMed
- Kim, S., Feinberg, B., Kant, R., Chui, B., Goldman, K., Park, J., Moses, W., Blaha, C., Iqbal, Z., Chow, C., Wright, N., Fissell, W.H., Zydney, A., Roy, S., Diffusive silicon nanopore membranes for hemodialysis applications. PLOS ONE 11, e0159526 (2016). PubMed
- Song, S., Faleo, G., Yeung, R., Kant, R., Posselt, A.M., Desai, T.A., Tang, Q., Roy, S., 2016. Silicon nanopore membrane (SNM) for islet encapsulation and immunoisolation under convective transport. Sci. Rep. 6, srep23679 (2016). PubMed
- Song, S., Blaha, C., Moses, W., Park, J., Wright, N., Groszek, J., Fissell, W., Vartanian, S., Posselt, A.M., Roy, S., 2017. An intravascular bioartificial pancreas device (iBAP) with silicon nanopore membranes (SNM) for islet encapsulation under convective mass transport. Lab. Chip 17, 1778–1792 (2017). PubMed
Cite as: N. R. Gough, Bionic Organs with Silicon Nanopore Membranes. BioSerendipity (11 September 2017) https://www.bioserendipity.com/bionic-organs-with-silicon-nanopore-membranes/