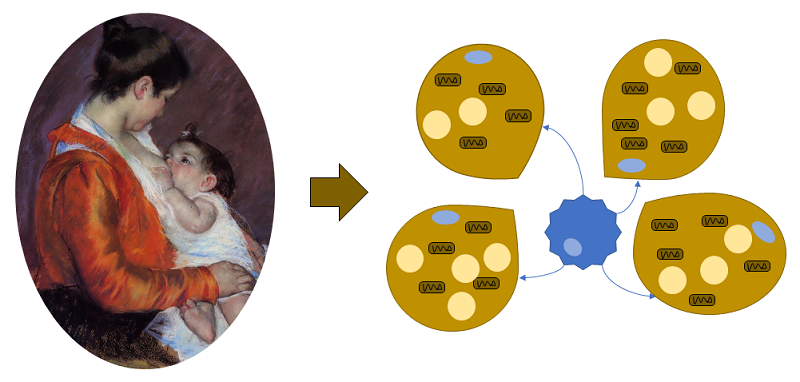
A recent article identified a role for alkylglycerol (AKG)-type ether lipids in human breast milk in the regulation of the development of fat tissue in nursing babies (Figure 1).
Intriguingly, the lipids affected tissue-resident macrophages in the fat tissue, adipose tissue macrophages (ATMs), not the adipose cells themselves. Using mice, Yu and colleagaues showed that the AKG lipids delayed the development of white adipose tissue (WAT) and retained beige adipose tissue (BeAT) (Figure 2), thus sustaining the thermogenic fat tissue during the period when newborns have limited ability to thermoregulate.

Furthermore, the increased proportion of BeAT in adipose tissue persisted even after weaning. These findings may explain why breastfed human children have reduced risk of developing obesity: Breast milk AKGs reduce the development of WAT and thus limit the amount of fat-storing adipose tissue.
A nutritionist or someone developing infant formula would consider the AKG lipids as non-essential nutrients needed for the baby’s development. A developmental biologist would see the AKG lipids as signaling molecules that regulate tissue development. How would an immunologist classify these lipids?
The infants ingest the lipids in breast milk. In the intestine, the lipids bind to chylomicrons and low-density lipoproteins and are absorbed by lymphatic vessels, which means that these nutrients bypass the liver and instead accumulate in adipose tissue. Feeding baby mice extra AKG delayed the transition of beige adipose tissue (BeAT) to white adipose tissue (WAT), and depriving the mice of these lipids (feeding them mouse formula) led to premature loss of BeAT. Compared with control mice, the mice receiving extra AKG lipids were better able to maintain core body temperature when exposed to hypothermic conditions. Even after weaning, the mice had less WAT mass without a change in overall weight, suggesting that supplementing with these lipids triggered a long term change in fat development. Breastfed human babies also had higher amounts of BeAT than those fed formula.
Surprisingly, studies with the adipocyte cell line 3T3-L1, which can differentiate into either cells with properties of the adipose cells in WAT or those in BeAT, showed that the fat cells themselves did not respond to added AKGs. For the added AKGs to trigger 3T3-L1 cells to turn on the BeAT genes, the cultures had to include both 3T3-L1 cells and ATMs. Even medium from ATMs exposed to AKGs was sufficient to stimulate the 3T3-L1 cells to turn on BeAT genes. Thus, the ATMs were producing a secreted molecule that controlled adipocyte gene expression.
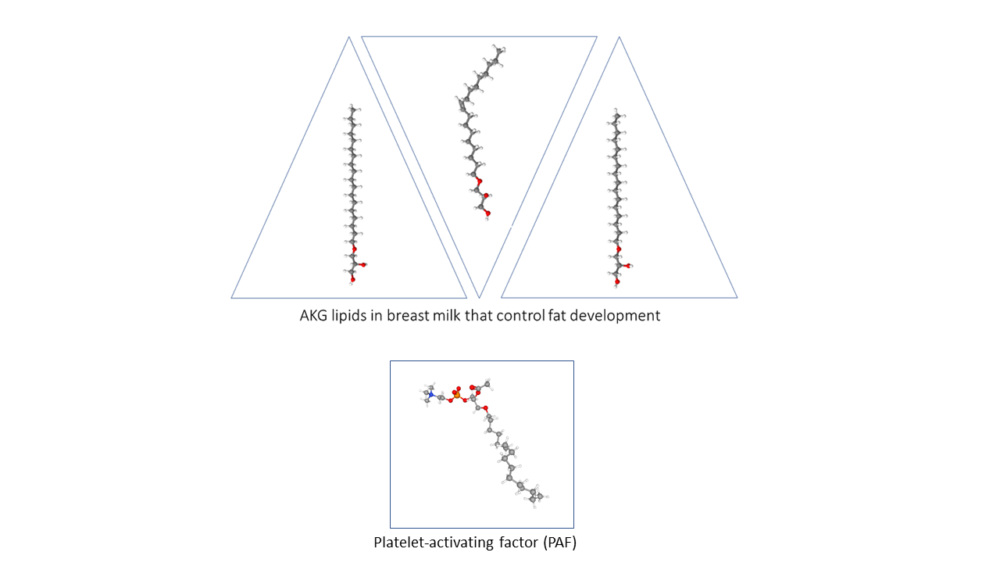
The researchers determined that the ATMs metabolized AKGs to produce the bioactive lipid platelet-activating factor (PAF) (Figure 3). Adipocytes degraded AKGs and did not produce PAF. When exposed to AKGs, both mouse and human ATMs produced PAF. However, PAF was not the signal that macrophages produced to influence fat cell differentiation: Exposing 3T3-L1 cells to PAF did not stimulate them to turn on BeAT genes. Instead, PAF was signaling to the macrophages (autocrine signaling) through the PAF receptor. Macrophages without this receptor did not respond to AKGs.

At this point, the pathway consists of ingested breast milk AKGs that are transported to the fat tissue, where they are metabolized by ATMs to produce PAF. PAF functions as an autocrine signal for the ATMs. What is the signal that ATMs produce to stimulate BeAT gene expression in fat cells? PAF stimulated ATMs to release the cytokine interleukin-6 (IL-6) (Figure 4).
Indeed, IL-6 stimulated BeAT gene expression in 3T3-L1 cells. IL-6 binds its receptor on the fat cells and activates a transcription factor called STAT3. 3T3-L1 cells cocultured with ATMs responded to AKG lipids with activation of STAT3. Thus, both IL-6 and AKGs activated STAT3 in fat cells, with AKGs requiring the presence of macrophages and IL-6 working in the absence of macrophages.
The researchers defined a complex signaling system from mother to child through breast milk lipids. The lipids regulated fat development by functioning as a substrate in the synthesis of PAF by the macrophages in the fat tissue. The macrophages responded to PAF by producing and releasing IL-6. The fat cells responded to IL-6 by turning on the genes needed for BeAT.
Intriguingly, adult mice with normal healthy weight did not respond to AKGs with an increase in BeAT. However, mice fed a high-fat diet that caused obesity did respond to AKGs with an increase in BeAT and a reduction in WAT. Thus, there is a switch that occurs in fat tissue. Here again, the key to this switch was the macrophages, not the fat cells themselves.
During the development of mice with normal weight, expression of the AGMO gene for the enzyme that degrades AKGs steadily increased in ATMs. At birth, this gene was not expressed, but by 14 days after birth (around the time of weaning), many ATMs expressing AGMO were present. The ATMs in the obese mice did not express AGMO. Thus, the macrophages respond to developmental signals or inflammatory signals associated with obesity to control the abundance of the enzyme that degrades AKGs. When this enzyme is abundant, the ATMs do not convert AKGs into PAF. When this enzyme is absent (at birth or in obesity), the ATMs convert AKGs into PAF, which in turn triggers IL-6 production that signals the fat cells to become BeAT.
These results have implications for producing infant formula that has the obesity-reducing effects of human breast milk: Formula should include AKGs. The results also have implications for understanding inflammatory changes associated with obesity and how these changes could be leveraged to treat obesity. Finally, these results challenge our ability to classify these lipids. Clearly, they are nutrients, because they are ingested and serve as substrates for enzymes that produce a bioactive molecule. However, they could also be considered signals themselves, because they relay a signal from mother to child.
Highlighted Article
H. Yu, S. Dilbaz, J. Coßmann, A. C. Hoang, V. Diedrich, A. Herwig, A. Harauma, Y. Hoshi, T. Moriguchi, K. Landgraf, A. Körner, C. Lucas, S. Brodesser, L. Balogh, J. Thuróczy, G. Karemore, M. S. Kuefner, E. A. Park, C. Rapp, J. B. Travers, T. Röszer, Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J. Clin. Invest. 129, 2485–2499 (2019). DOI: 10.1172/JCI125646 PubMed
Also of Interest
Cite as: N. R. Gough, Signal or Nutrient: Breast Milk Lipids in Macrophages. BioSerendipity (10 December 2019) https://www.bioserendipity.com/signal-or-nutrient-breast-milk-lipids-in-macrophages