Cellular and whole-body metabolism changes with age. An emerging idea is that preventing these metabolic changes may reduce negative effects of aging or at least delay them. Indeed, this idea is popular enough that companies are developing and selling dietary supplements intended to boost “healthy” metabolism. Many are intended to boost the amount of a molecule called nicotinamide adenine dinucleotide (NAD) or increase the activity of an NAD-dependent enzyme called SIRT1.
NAD+ in Metabolism
Like niacin (Vitamin B3) and nicotinamide, cells convert nicotinamide riboside (a compound in anti-aging supplements) into NAD, which exists in an oxidized form NAD+ and a reduced form NADH (Figure 1). NAD+ and NADH, the phosphorylated forms NADP+ and NADPH, and related molecules flavin adenine dinucleotide (FAD+ and FADH2), are key coenzymes or cosubstrates in metabolic processes that involve the chemical processes of oxidation and reduction. These kinds of reactions are collectively referred to as “redox” reactions. In an oxidation reaction, NAD+, NADP+, and FAD+ are oxidizing molecules, accepting a proton (H+) from another molecule in the reaction and are converted into NADH, NADPH, and FADH2, respectively; in a reduction reaction, NADH, NADPH, and FADH2 donate their protons (H+) to another molecule in the reaction and are converted back into NAD+, NADP+, and FAD+, respectively (Figure 1). In these reactions, the molecules are interconverted; the reducing or oxidizing forms are regenerated and can be used over and over in these kinds of reactions.
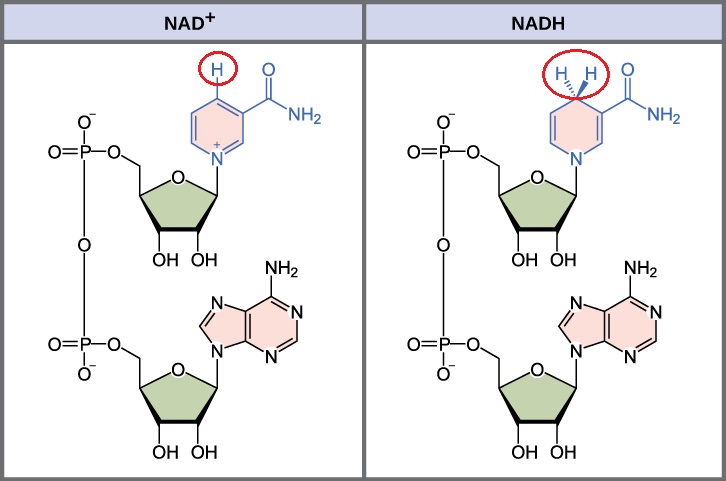
NAD+ and NADH are essential for cellular energy production, glycolysis (breakdown of glucose) to make energy, oxidation of fats (breakdown of lipids), and ATP synthesis by the mitochondria. NAD+ and NADPH also function in biosynthetic reactions, such as fatty acid and cholesterol synthesis. These are only a small sample of the hundreds of enzyme reactions in humans that have NAD+, NADH, NADP+, or NADPH as coenzymes. Thus, these molecules are critically important for cellular and organismal health. However, the balance of these coenzymes is also important with cells in different tissues requiring different amounts of each. Even different parts of cells have different requirements for an optimal balance of the oxidized and reduced forms of these molecules to maintain cellular metabolic processes.
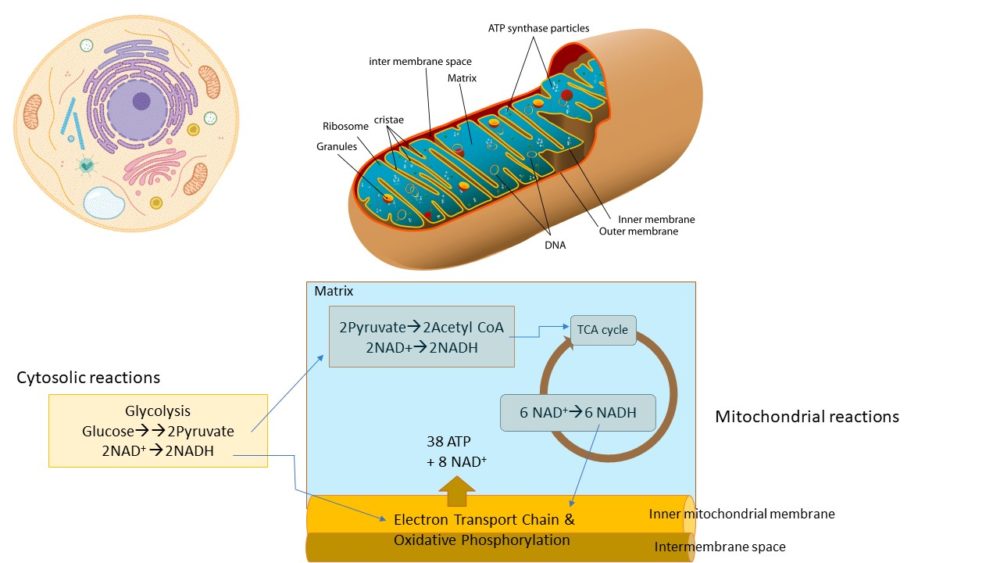
During the synthesis of ATP, the cell’s energy molecule, NAD and NADH are involved in all 3 parts of the process, which occur in the cytosol and in the mitochondria: Glycolysis (the breakdown of sugar), the Krebs cycle (also known as the TCA) cycle, and the electron transport chain and oxidative phosphorylation (Figure 2). Without NAD and NADH, these critical processes required for any form of cellular energy production cannot occur. During these reactions, NAD+ is not consumed and can be regenerated. However, there are other biochemical reactions in cells that involve enzymes that use up NAD+ during the reaction.
NAD+ is also a substrate in several posttranslational modification reactions (biochemical modifications of proteins) and in reactions that produce a cellular signaling molecule. In these reactions, NAD+ is consumed, not simply reduced into NADH. For the generation of the cellular signaling molecule, the enzymes that are cyclic ADP ribose synthases (CD38, BST1, SARM1) use NAD+ to make cyclic ADP-ribose (cADPR). cADPR stimulates the release of calcium ions (Ca2+) into the cytosol from intracellular compartments, such as the endoplasmic reticulum. Thus, cytosolic NAD+ is important in calcium signaling. CD38 and CD157 also metabolize NAD+, NR, and another NAD+ precursor; changes in the abundance of these enzymes may help the body maintain the proper amount of NAD+. The amount of CD38 increases with age. Because CD38 is involved in metabolizing and eliminating NAD+ and its precursors, the increase in CD38 with age could contribute to the decrease in NAD+ observed with age.
Through the action of poly-ADP ribose polymerases (PARPs), NAD+ is used to attach polymers of ADP-ribose onto various protein targets, most of which participate in cellular processes involving DNA. There are 17 human PARP genes that have different functions. PARPs accumulate at sites of DNA damage and promote repair, PARPs target proteins associated with DNA to promote access of telomerase to maintain the ends of chromosomes, and PARPs influence chromatin structure and target transcription factors to influence gene expression. Through PARPs, NAD+ has key roles maintaining DNA integrity and regulating gene expression.
PARPs couple DNA integrity to cellular metabolism through NAD+. Excess activity of PARPs, such as in cells with extensive and persistent DNA damage, can use so much of a cell’s NAD+ that the cell goes into a state of energy crisis and dies. This death is not necessarily bad. Cells with that much DNA damage may not recover and could become defective or cancerous. Would supplements that boost NAD+ overcome this essential regulatory mechanism and thus increase the risk of cancer?
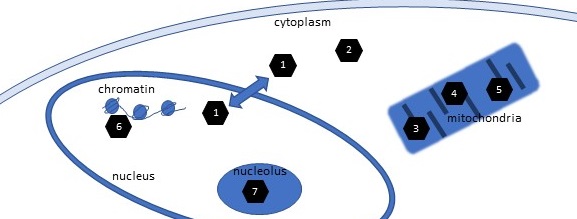
A final function of NAD+ is as a substrate in reactions mediated by enzymes of the sirtuin family. There are 7 sirtuin genes in humans. Their enzymatic activities include several posttranslational modification reactions. All sirtuins are NAD+-dependent protein deacetylases, which means that these enzymes use NAD+ to remove posttranslationally added acetyl groups attached to the amino acid lysine in proteins. When these acetyl groups are attached to histones, the proteins around which DNA wraps, then the removal of these groups inhibits gene expression by reducing the accessibility of the DNA to the proteins involved in gene expression. The members of the sirtuin family function in the cytoplasm, nucleus, and mitochondria of cells (Figure 3). They have diverse target proteins and regulate diverse aspects of cellular biology.
In addition to their deacetylase activity, many sirtuin family members also function in removing other molecules that can be attached to the lysine residues of proteins. Sirtuin 1 (SIRT1) is a demethylglutarylase; SIRT4 is a demethylglutarylase, hydroxymethylglutarylase, and 3-methylglutaconylase; SIRT5 is a demalonylase and desuccinylase; SIRT6 removes long chain fatty acids attached to proteins; and SIRT6 and SIRT4 both have ADP-ribosyltransferase activity (similar to PARP enzymes). These functions of sirtuins and the physiological importance of these posttranslational modifications are just beginning to be studied.
Challenges to Predicting the Effects of Changing the NAD+ Balance
With these diverse roles for NAD+, it is difficult to determine and predict how altering the NAD+/NADH balance will affect different people with different genetic backgrounds, diets, ages, and microbiomes. Cells in many tissues are highly sensitive to changes in metabolism. Many of the protein chemical modifications that are removed by sirtuins are related to intermediates in cellular metabolism. Changing the NAD+/NADH balance or the amount of NAD+ available in cells may not only directly affect the activity of the enzymes that rely on this molecule but, through its actions on cellular metabolism, may indirectly affect posttranslational protein modifications by changing the abundance of the metabolic intermediates that are attached to proteins to regulate their function.
Through these diverse protein modification, sirtuins help cells respond to changes in metabolism and regulate physiology in response to the normal daily variations in food intake and cellular activity states. Indeed, mammals exhibit a daily (circadian) cycle in NAD+ biosynthesis, which naturally alters the amount of available in NAD+. Through this circadian change, NAD+ affects mitochondrial metabolism through both sirtuins that function in mitochondria and the redox reactions that are part mitochondrial metabolism. How would taking a supplement that boosts NAD+ affect this physiological regulatory process?
Some types of T cells are affected by the amount NAD+. Studies found that conditions that increased the amount of NAD+, such as reduced abundance of CD38, promoted the anti-tumor activity of these Th1 and Th17 cells. This anti-tumor activity involved SIRT1. However, these types of T cells are also implicated in multiple autoimmune diseases. Thus, would chronically boosting NAD+ trigger autoimmune disease in susceptible individuals?
SIRT4 inhibits insulin secretion. Mice without this enzyme exhibit hyperinsulinemia and insulin resistance. Are there variations in the gene encoding this enzyme in humans that could be negatively affected by chronically increasing NAD+ and thus excessively stimulating SIRT4 activity?
Mitochondrial metabolism is also connected to the regulation of the gut microbiome (the microorganisms that colonize the gastrointestinal tract). Bacteria also rely on NAD+ for multiple cellular processes, including as a participant in redox reactions, an RNA modifier, and a substrate for enzymes that target DNA or proteins. The effect of long-term increase in NAD+ through dietary supplementation on the microbiome is unknown.
The research on NAD+and human health and disease is exciting. There may be conditions for which increasing NAD+ at specific times or for long periods may be beneficial. However, there may be people for whom boosting NAD+ triggers unwanted effects or has adverse interactions with medications. I suspect that our bodies have an optimal NAD+ set point and, try as we might, our bodies will maintain NAD+ at the proper balance for us. Providing supplements or NAD+ injections to boost NAD+ may trigger compensatory changes in the enzymes that metabolize NAD+, the enzymes that recycle its metabolites to regenerate NAD+, or the enzymes that synthesize this molecule from the amino acid tryptophan. Thus, likely many supplements, these may ultimately result in expensive pee as our bodies stimulate natural processes to eliminate the excess. Even coffee has enough niacin to trigger excretion of its metabolites.
Related Reading
Y. S. Elhassan, A. A. Philip, G. G. Lavery, Targeting NAD+ in metabolic disease: New insights into an old molecule. J. Endocr. Soc. 1, 816-835 (2017) DOI: 10.1210/js.2017-00092. PubMed
S. Johnson, S. Imai, NAD biosynthesis, aging, and disease. F1000Research 7 (F1000 Faculty Rev),132 (2018) DOI: 10.12688/f1000research.12120.1. PubMed
K. A. Anderson, M. F. Green, F. K. Huynk, G. R. Wagner, M. D. Hirschey, SnapShot: Mammalian sirtuins. Cell 159, 956.e1 (2014) DOI: 10.1016/j.cell.2014.10.045. PubMed
T. Nakagawa, L. Guarente, SnapShot: Sirtuins, NAD, and aging. Cell Metab. 20, 192.e1 (2014) DOI: 10.1016/j.cmet.2015.06.001. PubMed
S. Chatterjee, A. Daenthanasanmak, P. Chakraborty, M. W. Wyatt, P. Dhar, S. P. Selvan. J. Fu, J. Zhang, H. Nguyen, I. Kang, K. Toth, M. Al-Homrani, M. Husain, G. Beeson, L. Ball, K. Helke, S. Husain, E. Garrett-Mayer, G. Hardiman, M. Mehrotra, M. I. Nishimura, C. C. Beeson, M. G. Bupp, J. Wu, B. Ogretmen, C. M. Paulos, J. Rathmell. X.-Z. Yu, S. Mehrotra, CD38-NAD+ axis regulates immunotherapeutic anti-tumor T cell response. Cell Metab. 27, 85-100.e8 (2018) DOI: 10.1016/j.cmet.2017.10.006. PubMed
C. B. Peek, A. H. Affinati, K. M. Ramsey, H.-Y. Kuo, W. Yu, L. A. Sena, O. Ilkayeva, B. Marcheva, Y. Kobayashi, C. Omura, D. C. Levine, D. J. Bacsik, D. Gius, C. B. Newgard, E. Goetzman, N. S. Chandel, J. M. Denu, M. Mrksich, J. bass, Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342, 1243417 (2013) DOI: 10.1126/science.1243417. PubMed
K. A. Anderson, F. K. Huynh, K. Fisher-Wellman, J. D. Stuart, B. S. Peterson, J. D. Douros, G. R. Wagner, J. W. Thompson, A. S. Madsen, M. F. Green, R. M. Sivley, O. R. Ilkayeva, R. D. Stevens, D. S. Backos, J. A. Capra, C. A. Olsen, J. E. Campbell, D. M. Muoio. P. A. Grimsrud, M. D. Hirschey, SIRT4 is a lysine deacylase that controls leuicine metabolism and insulin secretion. Cell Metab. 25, 838-855.e15 (2017) DOI: 10.1016/j.cmet.2017.03.003. PubMed
J. I. Kremer, K. Gömpel, T. Bakuradze, G. Eisenbrand, E. Richling, Urinary excretion of niacin metabolites in humans after coffee consumption. Mol. Nutr. Food Res. 62, e1700735 (2018) DOI: 10.1002/mnfr.201700735. PubMed
Companies
Elysium Health (accessed on 12 June 2018) https://www.elysiumhealth.com/
ChromaDex (accessed on 12 June 2018) https://chromadex.com/Home/
Educational Resources
Cellular Respiration, Khan Academy (accessed 11 June 2018) https://www.khanacademy.org/science/biology/cellular-respiration-and-fermentation#intro-to-cellular-respiration
L. Chu, Energy of Living Systems, Copy of Biology. OpenStax CNX. (accessed on 12 June 2018) https://cnx.org/contents/[email protected]:dfq-SF-8@8/Energy-in-Living-Systems
Proteins that use NAD+, NADH, NADP or NADP+, BioCyc: Human Cyc (accessed on 8 June 2018) https://biocyc.org/HUMAN/substring-search?type=NIL&object=NAD&quickSearch=Quick+Search#ENZYME
Redox reactions involving NAD+, BioCyc: Human Cyc (accessed on 8 June 2018) https://biocyc.org/compound?orgid=HUMAN&id=NAD#tab=RXNS
Redox reactions involving NADH, BioCyc: Human Cyc (accessed on 8 June 2018) https://biocyc.org/compound?orgid=HUMAN&id=NADH#tab=RXNS
Redox reactions involving NADP+, BioCyc: Human Cyc (accessed on 8 June 2018) https://biocyc.org/compound?orgid=HUMAN&id=NADP#tab=RXNS
Redox reactions involving NADPH, BioCyc: Human Cyc (accessed on 8 June 2018) https://biocyc.org/compound?orgid=HUMAN&id=NADPH#tab=RXNS
Clinical Trials
Nicotinamide riboside, ClinicalTrials.gov (accessed 12 June 2018) https://clinicaltrials.gov/ct2/results?cond=&term=Nicotinamide+riboside
Pterostilbene, ClinicalTrials.gov (accessed 12 June 2018) https://clinicaltrials.gov/ct2/results?cond=&term=pterostilbene
Genes and Proteins
PARP gene family, Human Gene Nomenclature Committee (HGNC) (accessed on 8 June 2018) https://www.genenames.org/cgi-bin/genefamilies/set/684
Sirtuin gene family, Human Gene Nomenclature Committee (HGNC) (accessed on 8 June 2018) https://www.genenames.org/cgi-bin/genefamilies/set/938
CD38, Human Gene Nomenclature Committee (HGNC) (accessed on 8 June 2018) http://www.uniprot.org/uniprot/P28907
BST1, Human Gene Nomenclature Committee (HGNC) (accessed on 8 June 2018) http://www.uniprot.org/uniprot/Q10588
SARM1, Human Gene Nomenclature Committee (HGNC) (accessed on 8 June 2018) http://www.uniprot.org/uniprot/Q6SZW1
Cite as: N. R. Gough, Anti-Aging Supplements II: NAD+ as the Key to Healthy Aging. BioSerendipity (13 June 2018) https://www.bioserendipity.com/anti-aging-supplements-ii/
Also of Interest
Anti-Aging Supplements: The Basis for Basis
Anti-Aging Supplements III: Berries and Wine for Healthy Aging

