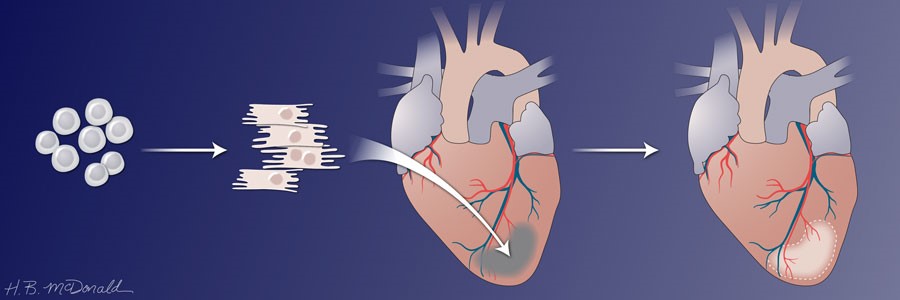
Heart muscle has limited ability to regenerate. This is why heart attacks cause permanent heart damage. A goal in cardiovascular medicine is to induce healing rather than scarring, especially in patients with large areas of heart damage that compromise heart function. One strategy under investigation is injecting immature heart muscle cells, called cardiomyocytes, into the injured area. The ability to produce cardiomyocytes in culture from either embryonic stem cells (ESCs) or from inducible pluripotent stem cells (iPSCs) and success with animal models have brought this strategy into clinical trials.
In 2014, Murry and colleagues stimulated human ESCs to form cardiomyocytes in culture, expanded the cells and froze the cells. After causing heart damage by blocking a blood vessel, the researchers thawed and injected the cells into the animals. The human cardiomyocytes were injected directly into the damaged area 2 weeks after the “heart attack.” The animals had to receive immunosuppressive drugs so that they did not reject the injected human cells. The damage in this study was relatively small. The results were impressive. In the one animal studied for 3 months, the human cardiomyocytes had integrated into the damaged area, the cells appeared to be maturing from the embryonic to the adult state, and blood vessels were growing into the area where the human cells had engrafted. Encouragingly, analysis of electrophysiological properties of the hearts showed that the human cardiomyocytes had electrically coupled to the monkey heart cells. Discouragingly, the animals receiving the human cardiomyocytes had arrhythmias. This was a very small-scale study with 7 animals, only one of which receiving the human cells was studied for 12 weeks. One control animal who received an injecting of the medium without any cells was studied for 2 weeks and another control animal for 4 weeks. One of the animals receiving the cells was studied for 2 weeks and the two others for 4 weeks.
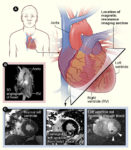
In 2018, Murry and colleagues performed a follow up study again using human ESCs induced to form cardiomyocytes and macaques as the model animal for heart repair. The goals were to determine if the human cardiomyocytes could restore contractile function and to better understand the arrhythmias observed in the previous study. To measure heart function and contractility, the researchers developed a method for performing cardiac magnetic resonance imaging (MRI) (Figure 2) on the monkeys. Among other indicators of heart health, this method enables measurement of left ventricular ejection fraction, a key measure of heart function and contractility. Different from the previous study, which involved a relatively small area of damage, this study involved a larger area of damage. Because the cells are human, the animals still required immunosuppressive drugs to prevent rejection of the injected cells. Again, the human cardiomyocytes were injected directly into the damaged area ~2 weeks after the experimentally triggered “heart attack.” Again, the number of animals in the study was small, so generalizing the results is difficult.
All of the animals had compromised heart function with reduced left ventricular ejection fractions after the induced heart damage. As before, in this second study, the human cells successfully engrafted with evidence of maturation to the adult state, and blood vessels grew into the graft. The 2 animals receiving cells and evaluated at 3 months had fully recovered left ventricular ejection fractions, whereas the control animal had a further decrease in heart function by this measure.
Electrophysiological properties and arrhythmias were assessed in 6 animals that received cells and 4 control animals. Both groups had increased occurrence of arrhythmias after the induced heart damage, but no statistically significant difference between the groups occurred throughout the duration of the study. Only one animal receiving cells developed severe ventricular arrhythmias. The difference in the frequency of the occurrence of arrhythmias in this study and the previous one appeared related to the difference in the size of the damage. However, this study suggested that control animals and animals receiving cells developed arrhythmias because of different types of abnormal electrical conduction. The control animals had arrhythmias arising from small site of reentry (the electrical activity stimulates the hearts cells in a place too soon), and animals receiving cells had arrhythmias caused by abnormal impulse generation (cells had abnormal pacemaker activity, the ability to set the heart rate).
A third study in 2016 performed similar experiments with macaques, but these researchers used iPSCs from immunologically matched, not genetically matched, monkeys instead of human ESCs. Shiba and colleagues isolated fibroblasts, genetically engineered the cells to make iPSCs, and then cultured the cells under conditions that converted them into cardiomyocytes. Similar to the studies with human ESCs, the cardiomyocytes were expanded, cryopreserved, then thawed before injection into the animals with experimentally induced heart damage. These animals still required immunosuppressive drugs to prevent rejection of the injected cells. The injected cells integrated with the animal’s hearts, formed heart muscle tissue, and became electrically coupled. The graft had new blood vessels. These researchers used a method called micro-computed tomography (μCT) to measure ejection fraction and also found that animals receiving the cells had improved heart function. All 5 animals receiving cells had arrhythmias 14 days after receiving the cells. In some of the animals, the arrhythmias appeared to resolve over time and were not present at 56 or 84 days.
Highlighted Articles
J. J. H. Chong, X. Yang, C. W. Don, E. Minami, Y.-W. Liu, J. J. Weyers, W. M. Mahoney, B. Van Biber, S. M. Cook, N. J. Palpant, J. A. Gantz, J. A. Fugate, V. Muskheli, G. M. Gough, K. W. Vogel, C. A. Astley, C. E. Hotchkiss, A. Baldessari, L. Pabon, H. Reinecke, E. A. Gill, V. Nelson, H.-P. Kiem, M. A. Laflamme, C. E. Murry, Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273 – 277 (2014). PubMed
Y.-W. Liu, B. Chen, X. Yang, J. A. Fugate, K. A. Kalucki, A. Futakuchi-Tsuchida, L. Couture, K. W. Vogel, C. A. Astley, A. Baldessari, J. Ogle, C. W. Don, Z. L. Steinberg, S. P. Seslar, S. A. Tuck, H. Tsuchida, A. V. Naumova, S. K. Dupras, M. S. Lyu, J. Lee, D. W. Hailey, H. Reinecke, L. Pabon, B. H. Fryer, W. R. MacLellan, R. S. Thies, C. E. Murry, Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hears of no-human primates. Nat. Biotechnol. 36, 597 – 605 (2018). PubMed
Y. Shiba, T. Gomibuchi, t. Seto, Y. Wada, H. Ichimura, Y. Tanaka, T. Ogasawara, K. Okada, N. Shiba, K. Sakamoto, D. Ido, T. Shiina, M. Ohkura, J. Nakai, N. Uno, Y. Kazuki, M. Oshimura, I. Minami, U. Ikeda, Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538, 388 – 391 (2016). PubMed
S. A. Fisher, C. Doree, A. Mathur, D. P. Taggart, E. Martin‐Rendon, Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database of Systematic Reviews 12, CD007888 (2016). DOI: 10.1002/14651858.CD007888.pub3 PubMed
S. A. Fisher, H. Zhang, C. Doree, A. Mathur, E. Martin‐Rendon, Stem cell treatment for acute myocardial infarction. Cochrane Database of Systematic Reviews 9, CD006536 (2015). DOI: 10.1002/14651858.CD006536.pub4 PubMed
Stem cells to repair heart damage? No so fast. Harvard Heart Letter (January 2018) https://www.health.harvard.edu/heart-health/stem-cells-to-repair-heart-damage-not-so-fast
Related Video
H. Al-Ghaili, Stem Cell Therapy Restores Heart Function Up To 90% After Heart Failure. Facebook/ScienceNaturePage (Season 11). Video https://www.facebook.com/ScienceNaturePage/videos/1387734764692111/UzpfSTEyNTUyMTQ1NDY6MTAyMTY3MDM5OTMzMzI5NjA/
Related Commentary
N. R. Gough, Scientific Misconduct Casts Doubt on Basis for a Stem Cell Clinical Trial. BioSerendipity (1 November 2018) https://www.bioserendipity.com/scientific-misconduct-casts-doubt-on-basis-for-a-stem-cell-clinical-trial/.
Cite as: N. R. Gough, Stem Cells Repair Injured Hearts. BioSerendipity (15 August 2018) https://www.bioserendipity.com/stem-cells-repair-injured-hearts/