Two studies in March 2019 focus on MYC as a target in cancer. MYC is encoded by a well known oncogene, meaning that it can promote cancer. However, MYC has both pro-cancer and anti-cancer properties. The anti-cancer activity tends to dominate when there is more of this protein in the cell; the pro-cancer activity dominates when there is less MYC. The first study by Harrington and colleagues tests the benefit of increasing MYC to promote its anti-cancer activity. The second study by Beaulieu and colleagues tests a mini-peptide based on MYC that interferes with MYC function.
Harrington and colleagues leveraged the difference cancer-related activities resulting from different amounts of MYC to design a combination therapy for Burkitt lymphoma. They increased the amount of MYC using two different strategies, and treated the cells with either doxorubicin (a DNA-damaging chemotherapeutic drug) or mapatumumab (a drug that activates a receptor that triggers cell death. The cells with higher amounts of MYC were sensitized to these drugs: They died at lower concentrations the doxorubicin or mapatumumab. Similar results were found using cancer models in mice. Combining a GSK-3 inhibitor (lithium or
CHIR99021) with doxorubicin increased cancer cell death compared to treatment with doxorubucin alone.
One of the strategies for increasing MYC abundance was treating the cells with an inhibitor of enzymes called GSK-3. These enzymes have multiple targets. Indeed, other studies have reported anti-cancer effects of inhibiting GSK-3, but those studies report mechanisms that do not involve MYC for the beneficial effects. Thus, the researchers performed experiments to verify that the increased sensitivity to the death-inducing effects of the other two cancer drugs related to an increase in MYC.
Some GSK-3 inhibitors are either approved for clinical use, such lithium for controlling bipolar disease, or were shown to be safe in clinical trials, such as tideglusib. This study indicates that, for chemotherapy-resistant Burkitt lymphoma, a strategically designed regimen that combines one of these inhibitors and a death receptor-activating drugs or traditional chemotherapeutic agents could be effective. Additionally, drugs or biologic agents that activate the death receptors, which had no anti-cancer benefit alone, may be effective in combination with a GSK-3 inhibitor. Thus, this study opens the door to several new options for treating resistant Burkitt lymphoma. How extensible these strategies are for other cancers remains unknown.
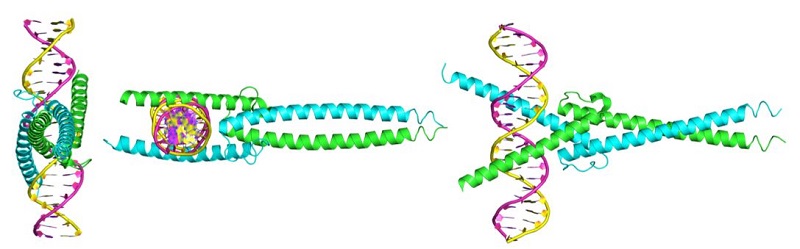
MYC itself is not an enzyme. It is a protein that interacts with DNA to regulate gene expression (Figure 1). MYC is also a member of a family of related proteins with overlapping functions. Finally, MYC interacts with other proteins and functions in the nucleus. Proteins with enzymatic activity or proteins with extracellular portions, like cell-surface receptors, tend to be easier to target pharmacologically. Targeting MYC directly has been challenging. The second study describes key pharmacological and pharmacokinetic properties of a “mini-protein” based on MYC. This 90-amino-acid mini-protein is called Omomyc, has 4 specific mutations compared to MYC, and inhibits some of the functions of MYC by interfering with the ability of MYC to interact with some binding partners. Indeed, Omomyc not only interferes with MYC but also with all members of this gene family. Omomyc blocks the interaction between MYC family members and interferes with genes that they activate, but Omomyc does not interfere with the repression of some MYC-regulated genes.
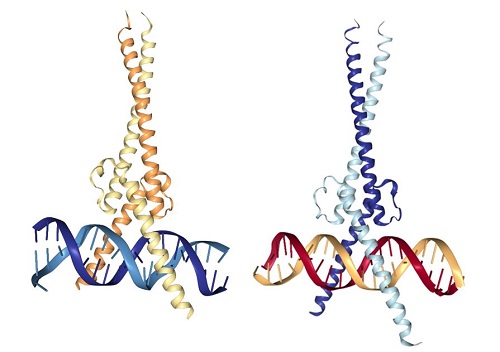
Figure 1. MYC-MAX and Omomyc dimers bound to DNA. [More details]
These researchers had previously used genetically engineered mice that expressed Omomyc to show that it had anticancer properties. However, it is not feasible to administer this agent by genetic engineering in patients. In the current study, Beaulieu and colleagues demonstrated that the mini-protein has cell-penetrating qualities, because of a part of the amino acid sequence adopts a structure called an amphipathic helix. Such sequences have cell-penetrating properties when attached to other peptides. In their study, the researchers tested the hypothesis that this sequence of Omomyc would enable the protein to enter into cells and inhibit MYC function.
A fluorescently tagged form of Omomyc entered nonsmall cell lung cancer cells in culture, and some signal was present in the nucleus, where the drug would function. Application of Omomyc to the cultured cells reduced their viability. Omomyc reduced the binding of MYC to gene promoters and altered the expression of MYC-regulated genes. Using a radioactively labeled form of Omomyc, the researchers showed that Omomyc delivered through the nose (intranasally) to mice reached the lungs within 30 minutes and persisted there for at least 48 hours. In a mouse model of lung cancer, Omomyc accumulated in the tumors and a 3-day or week-long treatment regimen reduced the number of proliferating cells in the tumors. Compared to control mice, a 4-week treatment blocked tumor growth, stimulated tumor cell death, reduced tumor grade, and increased the number of tumor-infiltrating T cells. Combining Omomyc with the chemotherapeutic agent paclitaxel prevented death during the 30-day experiment in a mouse model that uses patient-derived lung cancer cells.
This study by Beaulieu and colleagues demonstrates that peptides that interfere with specific protein domains, in this case the regions of MYC that let MYC bind to itself or its partners (dimerization) or to bind to DNA, represents an approach to inhibiting proteins that were considered “undruggable.” Additionally, this study suggests that Omomyc has properties that make it an effective medication even without any additional modifications. The mini-protein persists in tissues after a single administration, penetrates into cells, reaches the part of the cell where it needs to function (the nucleus), and effectively interferes with the function of the MYC family of proteins to reduce tumor progression. These findings will likely lead to rapid translation into clinical trials for lung and other cancers with aberrant MYC activity.
Together, these two studies show in preclinical models that MYC activity can be manipulated in opposite ways to achieve an anti-cancer response. How to leverage each approach to achieve the best benefit in patients remains to be determined.
Highlighted Articles
C. T. Harrington, E. Sotillo, A. Robert, K. E. Hayer, A. M. Bousz, J. Psathas, D. Yu, D. Taylor, C. V. Dang, P. Klein, M. D. Hogarty, B. Geoerger, W. S. El-Deiry, J. Wiels, A. Thomas-Tikhonenko, Transient stabilization, rather than inhibition, of MYC amplifies extrinsic apoptosis and therapeutic responses in refractory B-cell lymphoma. Leukemia 26 March 2019 DOI:
10.1038/s41375-019-0454-4 PubMed
M.-E. Beaulieu, T. Jauset, D. Massó-Vallés, S. Martínez-Martín, P. Rahl. L. Maltais, M. F. Zacarias-Fluck, S. Vasavuberta-Serra, E. Serrano del Pozo, C. Fiore, L. Foradada, V. C. Cano, M. Sánchez-Hervás, M. Guenther, E. R. Sanz, M. Oteo, C. Tremblay, G.
Martín, D. Letourneau, M. Montagne, M. A. M. Alonso, J. R. Whitfield, P. Lavigne, L. Soucek, Intrinsic cell-penetrating activity propels Omomyc from proof of concept to viable anti-MYC therapy. Sci. Transl. Med. 11, eaar5012 (2019). PubMed
Related Resources
M. Conacci-Sorrell, L. McFerrin, R. N. Eisenmann, An overview of MYC and its interactome. Cold Spring Harb. Perspect. Med. 4, a014357 (2014) DOI:10.1101/cshperspect.a014357. PubMed
MYC UniProtKB (as accessed on 2 April 2019) https://www.uniprot.org/uniprot/P01106
Cite as: N. R. Gough, Context matters for targeting MYC in cancer. BioSerendipity (3 April 2019)
https://www.bioserendipity.com/context-matters-for-targeting-myc-in-cancer/