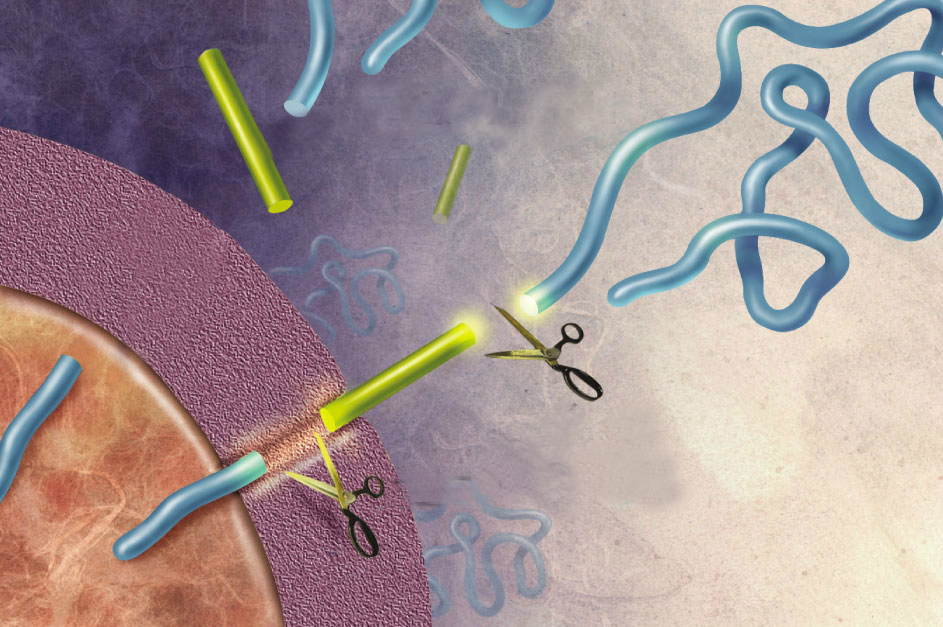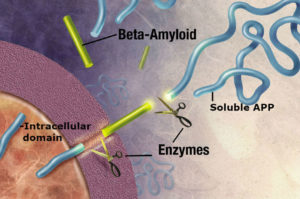
Amyloid precursor protein (APP) is best known as the protein that is cleaved to form the plaque-associated protein in the brains of Alzheimer’s patients. The APP gene encodes multiple alternatively spliced mRNA forms. The form that encodes a protein of 695 amino acids (APP695) is expressed in neurons. The transcript that encodes a 770 amino acid protein (APP770) is expressed in the cells that line the blood vessels, called vascular endothelial cells. The nonpathological roles of these proteins are less well understood. APP has a large extracellular portion, a transmembrane domain, and an intracellular portion. The variants can be cleaved to release most of the extracellular portion as a soluble form of APP (sAPP), a small fragment as the peptide Aβ (or beta-amyloid, the part of the protein that can form plaques), and an intracellular domain that can transport to the nucleus to regulate gene expression (Figure 1).
Sato and colleagues identified a role for the large soluble cleavage product of APP770 (sAPP770) as a signal that is released from vascular endothelial cells and limits the proliferation of neural stem cells (NSCs). Many tissue stem cells are located very close to the blood vessels that provide circulation to the tissue. These specialized vascular regions are called vascular stem cell niches. The endothelial cells and perivascular (cells surrounding the vasculature) provide signals that control when the stem cells become activated to help repair tissue injury or mediate tissue growth and when the stem cells should stay quiescent. When stem cells are quiescent, they are in a state akin to “sleep.” They are waiting in a relatively quiet state to be called to divide, renew, and differentiate. The study highlighted here shows that quiescence must be actively maintained. Without the right balance of signals, the stem cells can proliferate in appropriately.
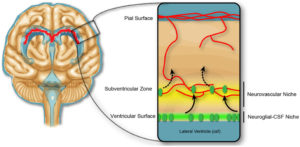
The researchers initially identified sAPP770 as an active component in present in medium in which a brain vascular endothelial cell line had been cultured (conditioned medium). When NSCs isolated from the subventricular zone of the lateral ventricle (Figure 2) from mouse brains were cultured either with this conditioned medium or sAPP770, the NSCs formed more numerous, but smaller, groups of cells called neurospheres. This resulted from a decrease in cell proliferation, not an increase in cell death.
To determine that sAPP770 came from the vascular endothelial cells in a live animal, the authors worked in mice to selectively knock out the gene for APP in endothelial cells or in astrocytes, which are one kind of cells that forms from the NSCs. Only the loss of APP from the endothelial cells increased the number of dividing NSCs in the mouse brain. Thus, sAPP770 represents a signal released from the vasculature the prevents excess and inappropriate NSC proliferation. This is just one of several signals produced by the vascular niche that regulates NSCs. Understanding these signals will help us understand not only the mechanisms that govern NSC behavior, which has implications for treating neurodegenerative disease and neural injury, but may also reveal how vascular niches control the activation of other tissue-specific stem cells that rely on these niches. Furthermore, this information may also provide insight into the signals that control whether a cancer cell, having left the primary tumor and traveled to the vascular niche in another site, remains quiescent at the vascular niche or divides and infiltrates the tissue, producing a site of metastasis.
Highlighted Research
Y. Sato, Y. Uchida, J. Hu, T. L. Young-Pearse, T. Niikura, Y.-S. Mukouyama, Solube APP functions as a vascular niche signal that controls adult neural stem cell number. Development 144, 2730-2736 (2017). PubMed
Cite as: N. R. Gough, Soluble Amyloid Precursor Protein Signals to Neural Stem Cells. BioSerendipity (5 February 2018). https://www.bioserendipity.com/soluble-amyloid-precursor-protein-signals-to-neural-stem-cells/