Protein kinases are enzymes that add phosphate groups onto specific amino acids on proteins. Most of these enzymes are active within cells. Kinases are often encoded by oncogenes, and aberrant activity of these regulatory enzymes is frequently associated with cancer. Data are emerging that kinases can also be released from cells. These secreted kinases can either stimulate phosphorylation of extracellular proteins or be internalized by nearby cells to phosphorylate targets within the cells that internalized the kinase. A pair of papers finds two distinct potentially cancer-promoting mechanisms of kinases released from cells.
Sánchez-Pozo and colleagues found that the tyrosine kinase c-SRC was released from cells and phosphorylated the extracellular protein metalloproteinase regulator TIMP2. Consequently, this increased the ability of TIMP2 to activate the enzyme MMP2, which degrades the extracellular matrix and releases some growth factors. Both of these activities of MMP2 can contribute to the migratory behavior of cancer cells and thus could enhance the ability of cancer cells to metastasize. Zhang and colleagues found that a specific isoform of the kinase S6K1 was released from cells and then internalized to phosphorylate intracellular targets in the recipient cells. The recipient cells exhibited increased growth (became larger), acquired the ability to form colonies in agar (an indication of tumorigenic behavior), and enhanced migration. Thus, these two kinases have roles beyond the cells that produce them and the released forms may have pro-cancer functions.
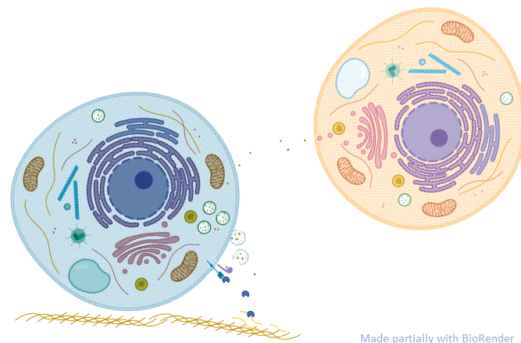
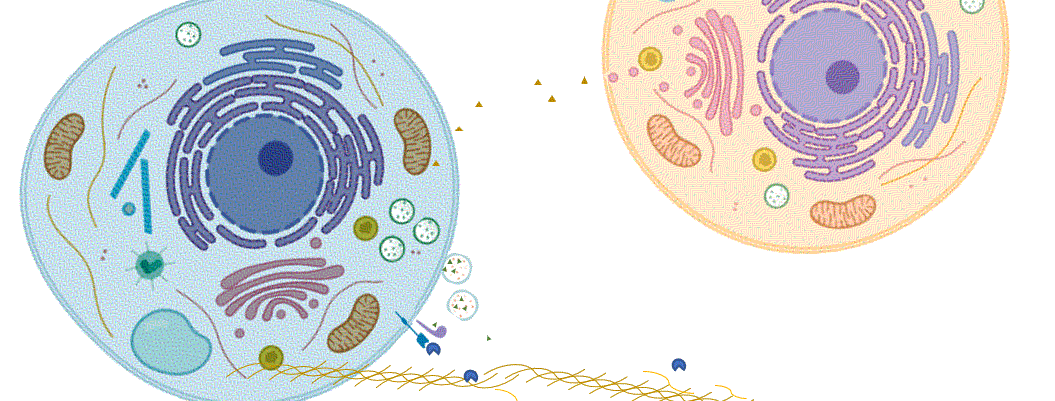
From a cell biology standpoint, these two examples are quite distinct (Figure 1). They each have different mechanisms of release and the location of their subsequent activity is different. Whereas c-SRC is released by cells through a mechanism that was not blocked by the chemical brefeldin A, release of the S6K1 isoform was blocked by this chemical. Brefeldin A inhibits proteins that control the movement of vesicles and their cargo through the endoplasmic reticulum (ER) and Golgi. Thus, cells appear to have at least two distinct mechanisms for releasing kinases. Sánchez-Pozo and colleagues proposed, although did not test, that the brefeldin A-independent mechanism involved the release of c-SRC in extracellular vesicles, such as exosomes. If that is the mechanism, then the question remains as to how was c-SRC released from the vesicles to act extracellularly on TIMP2 (as shown by the blue cell in Figure 1).
The S6K1 isoform (p85S6K) has a specific amino acid sequence (Met-Arg-Arg-Arg-Arg-Arg-Arg) at the N-terminus. The 6 arginine residues in this sequence enable proteins to penetrate cell membranes. Analysis of the entire human proteome found that p85S6K was one of 10 proteins with this 6-Arg sequence and the only protein with the sequence at the very N-terminus (Table 1). An isoform of the protein and lipid phosphatase PTEN (PTEN-L) was the only other protein in the list documented as released from cells to enter recipient cells and promote tumorigenesis. Release of p85S6K was inhibited by brefeldin A, suggesting that this protein passed through the ER and Golgi during its release from cells (as shown by the orange cell in Figure 1). In addition to a 6-Arg motif, p85S6K also has a nuclear localization sequence. The authors did not investigate whether this kinase was also localized to the nucleus in either the cells that produced it or in the recipient cells.
From a cancer standpoint, finding that kinases can be active extracellularly or taken up by nearby cells indicates the need to consider targets for kinases that are not within the cells expressing the genes encoding the kinases. Even if the cancer cell is expressing the kinase gene, the effects of the protein may be extracellular or occur in cells of the tumor microenvironment, if the kinase is released from the cancer cell. The S6K1 story also shows how important understanding not just genomic diversity but the diversity in the encoded protein products of genes. In humans, there are two S6K-encoding genes—S6K1 and S6K2. S6K1 encodes multiple protein products. Only p85S6K is released from cells, taken up by nearby cells, and promotes tumorigenic behaviors when applied to the medium of cells in culture. Unanswered questions remain regarding what other kinases are secreted, what mechanisms regulate kinase release, what physiological conditions rely on secreted kinases, and what roles secreted kinases play in disease.
| Protein | 6-Arg Motif Position | Total Length |
|---|---|---|
| PTEN-L | 47-52 | 576 |
| p85 S6K1 | 2-7 | 525 |
| BAHD1 | 581-587 | 780 |
| HNRNPA3 | 16-21 | 378 |
| MAGEE1 | 9-14 | 950 |
| NHLH1 | 66-71 | 133 |
| PPIG | 621-626 | 754 |
| RBM10 | 80-85 | 930 |
| SR-A1 (SCAF1) | 606-611 | 1312 |
| THEG | 122-127 | 379 |
Highlighted Articles
J. Sánchez-Pozo, A. J. Baker-Williams, M. R. Woodford, R. Bullard, B. Wei, M. Mollapour, W. G. Stetler-Stevenson, G. Bratslavsky, D. Bourboulia, Extracellular Phosphorylation of TIMP-2 by Secreted c-Src Tyrosine Kinase Controls MMP-2 Activity. iScience 1, 87-96 (2018). 10.1016/j.isci.2018.02.004 Full Text
J. Zhang, J. Guo, X. Qin, B. Wang, L. Zhang, Y. Wang, W. Gan, P. P. Pandolfi, W. Chen, W. Wei, The p85 isoform of the kinase S6K1 functions as a secreted oncoprotein to facilitate cell migration and tumor growth. Sci. Signal. 11, eaao1052 (2018). PubMed
Cite as: N. R. Gough, Secreted Kinases May Promote Malignancy. BioSerendipity (2 April 2018) https://www.bioserendipity.com/secreted-kinases-may-promote-malignancy