A type of immunotherapy uses antibodies that activate the TNF receptor family member CD40 on immune cells. CD40 is present on antigen-presenting cells (APCs) of the immune system and is activated by surface-bound proteins called CD40L. Activation of CD40 on APCs stimulates these cells to take up and display antigens to T and B cells, thereby stimulating an immune response. If the APCs display antigens present on tumor cells, then the subsequent T and B cell responses target the tumor cells. Application of antibodies that stimulate CD40 may increase the effectiveness of immunotherapies that target immune checkpoint inhibitors. Indeed, these antibodies may help convert an immune “cold” tumor into an immune “hot” target.
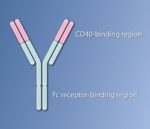
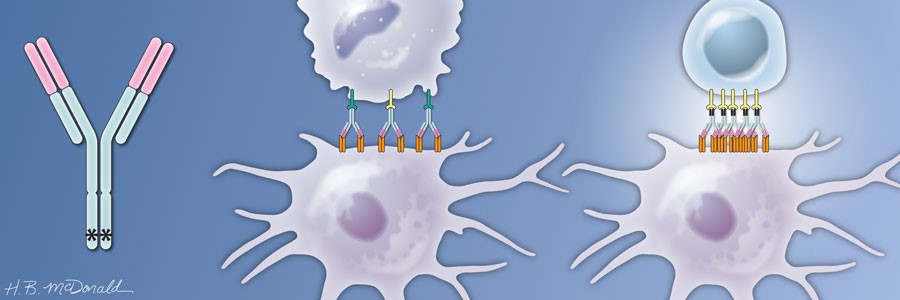
Based on this understanding of how the heavy chain of the antibody contributed to the anti-tumor immune response, Ravetch engineered a version of the antibody that had mutations in the heavy chain to optimize its interaction with FcγRIIB and minimize its interaction with FcγRIIA. The antibody is called APX005M, and the variable regions still interacted strongly with CD40. Because the amounts of FcγRIIB and FcγRIIA differs on the various types of immune cells, this engineered version of the CD40 antibody likely engages different immune cells to activate the CD40-positive APCs. The studies with the mice indicated that another benefit of APX005M was that this optimized antibody caused less adverse effects than the original CD40 antibodies that bound both Fc receptors. APX005M is now in clinical trials for several kinds of cancer, including those with poor prognosis (pancreatic cancer, melanoma, and brain cancers). The results of the initial Phase 1 safety trials are just beginning to be reported at conferences on cancer.
Intriguingly, this requirement for the antibody’s heavy chain to engage the Fc receptors appears to apply to many anti-cancer antibodies. Which Fc receptors the antibody binds affects how the antibody mediates an anti-tumor response. It is worth noting that the development of the optimized antibody from basic research into how the immune system recognizes and responds to antibodies took many years and involved the generation of an engineered mouse model. Another version of the mice with humanized Fc receptors was recently developed and is better suited for testing some aspects of cancer immunotherapies.
The knowledge of the complex interplay among the different cells of the immune system and how tumors avoid destruction is leading researchers to explore new treatment strategies. For example, giving a patient the CD40 antibody first so that the APCs re-activate T cells, then giving an inhibitor of the immune checkpoint to keep those T cells turned on may be more effective than simultaneous treatment or treatment with only one or the other type of immunotherapy.
Related Resources
Ravetch Laboratory, The Rockefeller University: https://www.rockefeller.edu/our-scientists/heads-of-laboratories/889-jeffrey-v-ravetch/ (accessed 5 September 2018)
Clinical Trials for APX0005M at clinicaltrials.gov https://www.clinicaltrials.gov/ct2/results?cond=&term=APX005M (accessed 5 September 2018)
F. Li, J. V. Ravetch, Inhibitory Fcγ receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science 333, 1030-1034 (2011). PubMed
P. Smith, D. J. DiLillo, S. Bournazos, F. Li, J. V. Ravetch, Mouse model recapitulating human Fcγ receptor structural and functional diversity. Proc. Natl. Acad. Sci. U.S. A. 109, 6181–6186 (2012). PubMed
F. Li, J. V. Ravetch, Apoptotic and antitumor activity of death receptor antibodies require inhibitory Fcγ receptor engagement. Proc. Natl. Acad. Sci. U.S. A. 109, 10966-10971 (2012). PubMed
F. Li, J. V. Ravetch, Antitumor activities of agonistic anti-TNFR antibodies require differential FcγRIIB coengagement in vivo. Proc. Natl. Acad. Sci. U.S. A.110, 19501-19506 (2013). PubMed
D. J. Dilillo, J. V. Ravetch, Differential Fc-receptor engagement drives an anti-tumor vaccinal effect. Cell 161, 1035-1045 (2015). PubMed
R. Dahan, B. C. Barnhart, F. Li, A. P. Yamniuk, A. J. Korman, Therapeutic activity of agonistic, human anti-CD40 monoclonal antibodies requires selective FcγR engagement. Cancer Cell 29, 820-831 (2016). PubMed
P. Bruhns, J.-L. Teillaud, Inhibitory IgG receptor-expressing cells: The must-have accessory for anti-CD40 immunomodulatory mAb efficacy. Cancer Cell 29, 771-773 (2018). PubMed
E. Casey, S. Bournazos, G. Mo, P. Mondello, K. S. Tan, J. V. Ravetch, D. A. Scheinberg, A new mouse expressing human Fcγ receptors to better predict therapeutic efficacy of human anti-cancer antibodies. Leukemia 32, 547 (2018). PubMed
R. H. Vonderheide, The immune revolutions: A case for priming, not checkpoint. Cancer Cell 33, 563-569 (2018). PubMed
Cite this article:
N. R. Gough, Optimizing anti-tumor therapies with engineered antibodies. BioSerendipity (18 September 2018) https://www.bioserendipity.com/optimizing-anti-tumor-therapies-with-engineered-antibodies/
Also of Interest in BioSerendipity