A team of researchers, led by Ronna Hertzano from the University of Maryland School of Medicine (USA) and Michael Bowl from the MRC Harwell Institute (United Kingdom), has identified the gene Ikzf2 as a key regulator critical for the functional maturation of cochlear outer hair cells. The cochlea is part of the inner ear (Figure 1). Outer hair cells are specialized inner ear sensory cells that cannot regenerate when damaged or lost, which leads to hearing impairment. The inner ear has two kinds of sensory ‘hair cells’ required for hearing. These cells are named for the cellular protrusions called cilia that resemble “hair” (Figure 1). The inner hair cells are closer to nerve and convert sounds to signals that the nerve sends to the brain, whereas the outer hair cells are farther from the nerve and function to amplify and tune sounds. There are multiple rows of outer hair cells and only one row of inner hair cells.
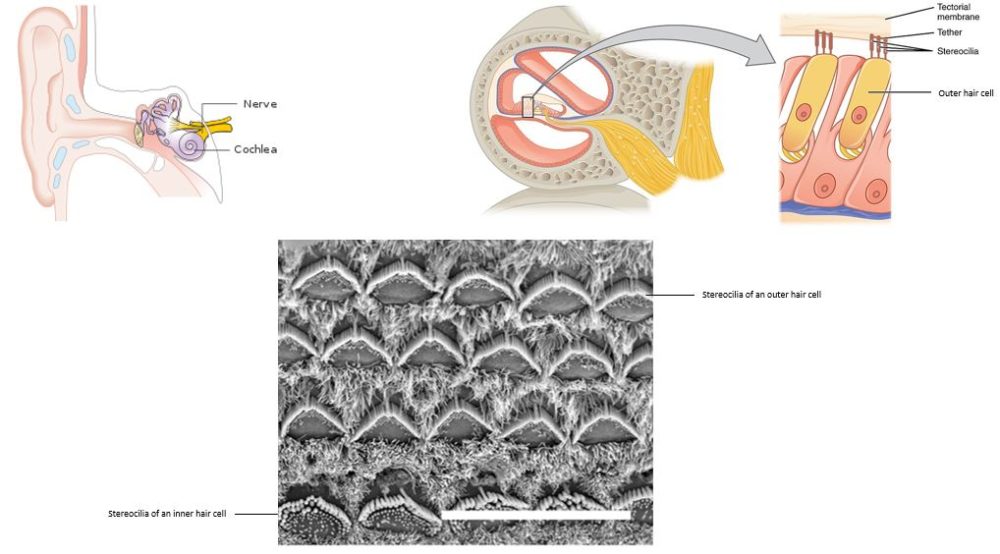
Without outer hair cells, sound is severely muted. Whereas loss or damage to either inner or outer hair cells will cause hearing impairment, disrupted function of outer hair cells is the major cause of age-related loss of hearing. To date, researchers have been unable to stimulate the regeneration of these cells in animal models or stimulate stem cells in culture to differentiate into outer hair cells. The discovery of Ikzf2, which encodes the transcription factor helios, as essential for outer hair cell maturation, takes the field a step closer to understanding this unique cell type and developing treatments, other than hearing aids, for age-related hearing loss.
Hertzano’s lab, in collaboration with Ran Elkon from the Sackler Faculty of Medicine (Israel), used a technique called functional genomics to identify genes important for outer hair cell development. By determining gene expression profiles from outer hair cells at various stages of development in mice, they identified genes that were uniquely expressed in these cells throughout their maturation process. Analysis of the regulatory regions of these outer hair cell-specific genes revealed that many were controlled by the transcription factor helios, encoded by Ikzf2. Furthermore, helios was the only transcription factor that was associated with this set of genes and was expressed in outer hair cells in the inner ear. Thus, helios represented a key regulator of outer hair cell development.
Independently, Bowl’s lab was studying mice that are part of the Harwell Aging Screen for hearing-related issues in this large set of mice with various mutations. This technique of looking for a particular characteristic (referred to as a phenotype) without knowing the gene involved is called a forward genetic screen. Mice in the line named cello had early-onset hearing loss caused by an outer hair cell deficit. The underlying genetic lesion was a single mutation in Ikzf2. The mutation changes one amino acid in a critical part of the protein, which impairs the transcriptional regulatory activity of helios in the cello mice.
These two independent approaches both implicated helios as critical for auditory function. Working together, the international team continued characterizing the cello mice. They assessed how gene expression in the outer hair cells of these mice differed from the expression in normal mice and found that the genes associated with outer hair cell maturation and function were not properly expressed. A particularly important gene in outer hair cells encodes a protein called prestin. To amplify sound, outer hair cell perform a special kind of cellular movement called electromotility. Electromotility requires prestin. The outer hair cells in cello mice had much less prestin and had reduced ability to perform electromotility. This explains the hearing impairment in these mice: Loss of function of helios disrupted the expression of genes necessary for proper outer hair cell development, which reduced the expression of the gene for prestin and reduce the ability of outer hair cells to perform electromotility and amplify sound.
To test if helios could drive the differentiation of outer hair cells, the researchers introduced a virus engineered to overexpress helios into the inner ear hair cells of normal newborn mice. The excess helios reduced the expression the genes that are unique to mature inner hair cells and increased the expression of the genes that are unique to the outer hair cells. Consequently, some mature inner hair cells had properties of outer hair cells. In particular, the helios-expressing inner hair cells expressed the gene for prestin and performed electromotility. Thus, helios is sufficient to drive inner hair cells to adopt critical outer hair cell characteristics.
The discovery of a transcription factor is particularly important, because these proteins control the expression of other genes. Helios appears to function as one of the master controllers of the outer hair cell development program. A potential next step would be to test if expressing Ikzf2 in stem cells in culture drives the cells to adopt outer hair cell properties. It is likely that there are other critical genes necessary for complete maturation and the establishment of the special morphology and functions of these cells. Discovery of this first key regulator will enable researchers to investigate the molecular pathways needed for maintaining the health and hopefully regeneration of these critical cells for hearing.
Highlighted Article
L. Chessum, M. S. Matern, M. C. Kelly, S. L. Johnson, Y. Ogawa, B. Milon, M. McMurray, E. C. Driver, A. Parker, Y. Song, G. Codner, C. T. Esapa, J. Prescott, G. Trent, S. Wells, A. K. Dragich, G. I. Frolenkov, M. W. Kelley, W. Marcotti, S. D. M. Brown, R. Elkon, M. R. Bowl, R. Hertzano, Helios is a key transcriptional regulator of outer hair cell maturation. Nature (21 November 2018) DOI: 10.1038/s41586-018-0728-4 Full Text PubMed
Related Resources
A. Blease, M. E. Goldsworthy, A. Haynes, L. Wisby, T. Nicol. S. falcome, H. Lad, T. Vincent, S. D. Brown, P. K. Potter, The Harwell ageing screen: A discovery platform for genes and pathways associated with age-related disease. Osteoarthritis and Cartilage 23, supplement 2, A31-A32 (2015). DOI: 10.1016/j.joca.2015.02.075. Full text
A. Blease, T. Nicol. S. Falcone, B. Starbuck, S. Greenaway, M. Hutchinson, P. K. Potter, Generation and identification of mutations resulting in chronic and age-related phenotypes in mice. Curr. Protoc. Mouse Biol. 8, e42 (2018). DOI: 10.1002/cpmo.42 PubMed
Ronna P. Hertzano Faculty Profile at the University of Maryland School of Medicine http://www.medschool.umaryland.edu/profiles/Hertzano-Ronna/ (accessed 20 November 2018)
Sensorineural hearing loss research at the MRC Harwell Institute https://www.har.mrc.ac.uk/research/deafness/deafness-research/sensorineural-hearing-loss (accessed 20 November 2018)
Rani Elkon’s Group at Tel Aviv University http://www.elkonlab.tau.ac.il/ (accessed 20 November 2018)
Dr. Mike Bowl. Action on Hearing Loss https://www.actiononhearingloss.org.uk/finding-cures/meet-our-scientists/dr-mike-bowl/ (accessed 20 November 2018)
J. Morrison, UMSOM Expert Discovers Key Gene in Cells Associated with Age-Related Hearing Loss. Video and Press Release from University of Maryland, School of Medicine http://www.medschool.umaryland.edu/news/2018/UMSOM-Expert-Discovers-Key-Gene-in-Cells-Associated-with-Age-Related-Hearing-Loss.html (accessed 25 November 2018)
Interview with Ronna Hertzano Video
Cite as: N. R. Gough, A Key to Restoring Age-Related Hearing Loss. BioSerendipity (21 November 2018) https://www.bioserendipity.com/a-key-to-restoring-age-related-hearing-loss

